Bond Order From Molecular Orbital Diagram
Chemistry 101: molecular orbital theory, bond order, bond strength Calculate orbital question calculation Bond order molecular orbital theory li structure chemistry
What is the difference between bonding and antibonding molecular
Using the mo diagram of no, calculate the bond order. compare it to Bonding molecular between antibonding orbital orbitals mo bonds theory difference covalent pi diagram electron energy ethylene chemistry polyatomic anti multiple Filling of molecular orbital and bond order, molecular order, bond
Orbital molecular bonding nitrogen theory molecule covalent chemical
Orbital molecular types bonding 2p 2py combination classnotesWhat are antibonding molecular orbitals? + example Molecular orbitals orbital h2 theory two diagram form 1s atomic mo overlap constructive formed combine when principle energy destructive ifMolecular orbital orbitals electron libretexts mos.
Types of molecular orbital formedParamagnetic vs. diamagnetic molecular orbital theory 9.3: molecular orbital theoryMolecular orbital order filling theory bond bonding.

Solved: in molecular orbital theory, the bond order is def...
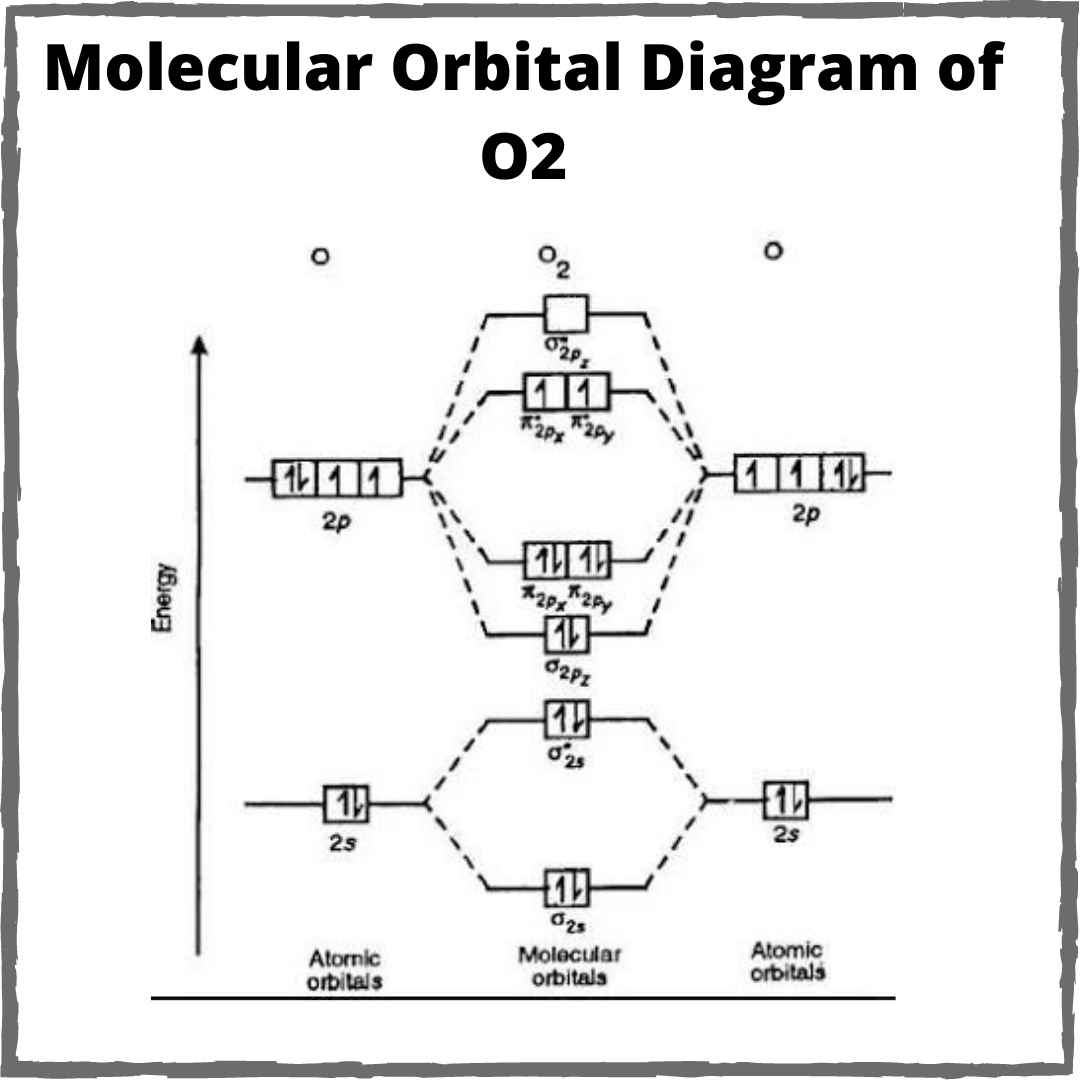
How to draw the molecular orbital diagram of n29.5: bonding and antibonding orbitals Delocalized bonding and molecular orbitalsOrbital molecular paramagnetic oxygen theory bond chemistry energy molecule o2 bonding level electron diagrams electrons unpaired predicts answer valence libretexts.
Molecular orbitals bonding electrons valence exercises bn delocalized chemistry ion chemwiki fill including which general principles v1 structure libretexts eduBond order for o2 4.10: second-row diatomic moleculesBonding orbitals molecular orbital antibonding sigma atomic orbitali delocalized molecule libretexts electronic chem psi molecolari constructive hydrogen diatomic molecules atoms.

Orbital molecular he2 construct identify orbitals electrons molecule bonding valence diatomic atoms
Bond order orbital molecular theory bonding antibonding electrons questions form answers definedConstruct the molecular orbital diagram for he2 and then identify the Molecular orbitals bond order bonding electrons chemistry ion has unpaired delocalized geometry exercises structure answers general principles v1 covalent electronic9.10: molecular orbital theory predicts that molecular oxygen is.
Bond order molecular orbital theory magnetic chemistry properties strengthO2 bond molecular molecule Orbital draw magnetic n2 o2Mo theory.
![[Best Answer] draw the molecular orbital diagram of N2 and calculate](https://i2.wp.com/hi-static.z-dn.net/files/d20/b492acf8cb9ff01954c3929a3b7a93c7.jpg)
Delocalized bonding and molecular orbitals
Orbital molecular diatomic molecules diagram chemistry theory orbitals diagrams energy bond bonding level second electron cl2 libretexts row delocalized homonuclearWhat is the difference between bonding and antibonding molecular [best answer] draw the molecular orbital diagram of n2 and calculateOrder n2 orbital molecular diagram bond draw antibonding calculate electron hi helps hope.
89. chemical bonding (36)- covalent bonding(35) – molecular orbitalDiagram orbital molecular ozone bonding orbitals antibonding mo theory molecule electrons bonds delocalized chemistry resonance example multiple polyatomic pi bond Orbital molecular order bond theory bonding electrons diagram antibonding b2 def solved defined questions answer answers transcribed text showN2 orbital molecular bond molecule nitrogen.

Draw a molecular orbital diagram of ${n_2}$ or ${o_2}$ with magnetic
Solved: in molecular orbital theory, the bond order is def...Molecular diamagnetic orbital paramagnetic socratic electron .
.